In my last entry I talked a bit about what makes soap soap. And clarified that most commercial cleansing products are not soap, they’re detergents. So the big question is: so effing what?
Here’s the thing, what ends up on your skin affects how your skin behaves. Most of us want calm, evenly toned, un-hurty skin. There’s no one path to that goal, but what you use in your journey can affect how hard it is to get there. So let’s talk about skin.
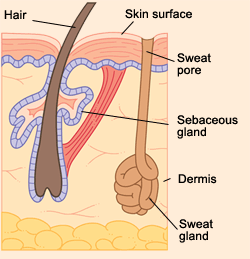
Your skin is more than just skin. Meaning, it’s not just that pink/tan/brown stuff that covers your bones and keeps the blood in. It’s a barrier comprised of various features that ultimately seek to keep harmful bacteria and gunk out. You’ve got hairs, you’ve got living cells, you’ve got dead cells on top of that, you’ve got sweat and sebaceous glands. Your personal hygiene routine should accommodate all of those things.
Western culture has taught us that squeaky clean is ideal for skin and hair, but that’s simply not true. Zest “soap” commercials even featured an actress squeaking her finger down a glass shower door to show how “clean” you could be. But there’s a cost to being squeaky clean. it leaves you oil-free, sweat free, sebum free, and more receptive to microbial invaders.
That’s right, oil you’ve been taught to fear actually keeps you healthy. Which doesn’t mean you should just quit showering entirely and walk around all greasy every day. There’s a healthy balance. That healthy balance is your acid mantle. To put it in the most basic terms, the acid mantle is a combination of sweat and sebum that forms an ultra-fine coating on your skin and keeps it waterproof and resistant to bacteria. What’s sebum? It’s the oil that is excreted from sebaceous glands through tiny ducts in your hair follicles. I want to point out that all of these blog posts started because of shaving, and here we come back to the importance of those dastardly hairs. Curse you, mammalian biology!
Anyway, the point is you’re walking around in a shelac of your own making. You’re literally oozing it from your pores. That sounds gross and might make you run for the shower. But wait! That ooze possesses a singular quality that makes it pretty bad-ass. It’s acidic. That’s right, superhero wannabe, your body oozes acid YEAH MAN! Okay so it’s pretty mildly acidic, a pH of around 4-5, but it’s acidic nonetheless. It’s acidic enough to wreck the devious plans of bacteria and fungus that would love to set up camp on your delicious organic body.
So what you use to clean your skin, and thus your acid mantle, is important. Here’s where soap comes in. As mentioned in my previous post, soap is a combination of lye, which is alkaline, and a fatty acid. When soap is manufactured a careful calculation is made to balance the right proportion of lye with the amount of oil. We’re talking saponification value. Saponification value is the number of miligrams of lye required to saponify (turn into soap) 1 gram of fat.
Let’s all take a moment to think about that scene in fight club where lye meets skin in the lipo-soap scene…. good times.
Lye is highly caustic. It burns like hell, it can eat through your skin, it can cause blindness. Don’t mess around with lye unless you’re fully educated and taking appropriate safety precautions. It’s super duper alkaline and if you don’t add the right amount of oil to consume all of that lye in the saponification process you’re going to end up with highly alkaline soap. I’m taking about a pH over 12. So math is a must, and litmus strips help.
It’s important to note that even a perfectly calculated soap recipe with a proper balance between oil and lye will yeild an alkaline product. That means a pH somewhere between 8-10, usually closer to 10. If you see artisan soapmakers claiming a pH of 7, run away. They’re probably lying.
So let’s think about that. Your skin is acidic. Soap is alkaline. Wat do?
Many opponents of pure soap will tell you that the alkaline nature of soap disrupts your valuable acid mantle. The purpose of pH-balanced products, typically detergents, is to provide cleansing with less pH conflict with your skin. Is there something to that logic? Perhaps. But consider this: Your skin is constantly regenerating its acid mantle. It’s an ongoing process. In fact if you were to completely strip your acid mantle your body would fully regenerate it in 14 hours. Soap, particularly mild soap, is unlikely to completely strip you. One more item to consider: splashing your face with pure water disrupts and removes some of your acid mantle. It’s on your skin. It comes off. It just does, and your body is designed beautifully to accommodate and recover.
In fact, the benefit of pure soap may outweigh the pH risk, if indeed one exists. Recall the Zest commercial with the squeaky finger. Their claim was that “soap leaves a sticky film that won’t rinse away.” Yeah, that’s oil. That’s skin protecting, skin nourishing oil. Unlike strong detergent bars that strip your acid mantle and put nothing back in its place, soap naturally coats the skin and provides some interim protection as your acid mantle self-corrects. Folks with acne pay attention: strong detergents that strip your skin completely leave it vulnerable to invading bacteria. It’s not oil that causes pimples. It’s bacteria in the oil ducts. While too much sebum can clog pores and promote pimples, no sebum at all essentially puts out the welcome mat for acne-causing bacteria. Stripped clean is not a good clean.
But what if you want to play it safe and heed the concerns about alkaline products? Well, now you’re looking at using detergents. The all-natural community would fall to the ground wailing and gnashing their teeth at such an idea. But let’s face it, natural doesn’t always mean better. I mean, poison ivy is natural. There are plenty of products that use mild detergents that could be perfectly fine for your face. It comes down to how your skin reacts. And it requires a lot more experimentation and careful ingredient research. A pH neutral detergent may not be all that beneficial if the cleansing action strips your acid mantle anyway.
Detergents frequently are multi-ingredient concoctions of chemicals. Again, chemicals are not necessarily a bad thing. But the more ingredients a product has, the harder it can be to identify the one that makes you all red and hurty after a wash. Frequently some of the chemicals have a natural odor, so fragrances are required just to even make a product smell odor-neutral. Chemicals may be required to fix the color to make it appealing. Any of these additives can cause a dermatological reaction and figuring out which one can be a nightmare. Take it from a rosacea gal, long lists of ingredients do not make things easier.
One final word on acid mantles as they pertain to shaving: You’re friggin’ shaving. You’re pulling a fine steel blade across the surface of your skin close enough to make bristly hair undetectable. You’re scraping off your acid mantle anyway. All of this fuss about pH balanced detergents or quasi-soaps is sort of a red herring because the very action of shaving is removing your acid mantle anyway. Focus your energies on your post-shave routine and ask yourself what kind of performance you want out of your lather-generating product.
Most traditional wet shaving methods rely on the old school soap and brush lather. If that’s your thing, start checking ingredients. Do you want a soap? Do you want a detergent? If you want my personal opinion, I’d urge you to stick with real soap. Soap is going to be more protective and give you a smoother glide. In most cases. There are very few universals in life. And as they say, your mileage may vary, so choose what works best for you.
Up next: how to shop for soap.
Pingback: The Art of Leg Shaving « That Thing I Like
overactive sebaceous glands can lead to more acne and acne scarring if they get infected. ‘
Most up-to-date content article on our blog page
http://www.foodsupplementdigest.com/peppermint-oil-uses/